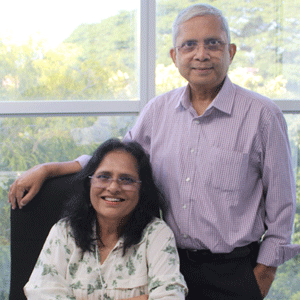
Clinical research is evolving. Digital transformation and strategic realignment impressed upon by cost pressures, complexity, and patient demands are shaping the sector’s future. Technological advancements decentralized clinical trials, personalized medicine, and patient-centricity are guiding the direction of research, propelled by the notion of minimizing burden and enhancing outcomes. Modern CRO’s need to upskill consistently, as the Indian and global markets refine and demand across industries like pharmaceuticals, and biopharmaceuticals elevates.
Within India’s changing clinical research landscape, Nextvel has emerged as a trusted partner. Guiding clients through the evolving rules and regulatory shifts, the Contract Research Organization, renowned for facilitating clinical research with novel therapies, supports clients to adapt to the industry shifts. From simplifying client transitions from outdated manual processes to electronic systems, laying down the regulatory pathways, to leveling up facilities aligned with cGMP guidelines, the company supports clinical development progress at multiple stages.
Diversified Expertise
Nextvel’s edge stems from its huge spectrum of services designed for the pharmaceutical, biotechnology, and medical device industries. The firm handles solutions throughout the drug and device development lifecycle, helping clients achieve greater efficiency, reduce costs, and expedite a journey of an innovation seamlessly from discovery to commercialization.
The firm’s service range encompasses project management, clinical operations, regulatory affairs, medical writing, data management, statistics, and quality assurance services for phase I to IV trials. It also offers support for the establishment of quality management systems aligned to the industry standards and client requirements.
“We engage with clients earlier on in the life cycle and help them navigate those unique regulatory requirements, help in coordination with the pre-clinical studies and help them with robust and comprehensive regulatory dossier preparation”, shares Mala Srivastava, Co-Founder & Managing Partner, Nextvel Consulting.
Nextvel stands out for its specialization in therapeutic areas such as cell gene therapy products, rare diseases, medical devices, and In vitro Devices. Beyond this, the firm also provides end-to-end handholding for clients that depends upon specialized processes for seamless and precise operations.
Shifting from the standard one-size-fits-all solutions, Nextvel’s flexible engagement models ensure client needs are accurately met. Operational excellence, speed, and global reach with local insight with a strong commitment to ethics, integrity, and quality underline the firm’s foundational values.
“Our commitment to quality as an ISO 9001:2015 certified and CDSCO registered organization is evident at every position. Our risk-based strategy, commitment to championing quality and regulatory compliance, collaborative partnerships, Quality by Design model, agile governance frameworks, and automation implementation help us achieve desired quality standards”, adds Akash Srivastava, Co-Founder & Managing Partner, Nextvel Consulting.
Future Roadmap
Emphasizing holistic growth, the firm’s focus remains on innovative therapies, AI/ML-based healthcare solutions, and more, as well as embracing new models of clinical research. Technology integrations for streamlining trials, building trust through continuous quality improvements remain consistent.
With the vision to keep innovation accessible, Nextvel is on the journey to redefine excellence in CRO services across the world.
We use cookies to ensure you get the best experience on our website. Read more...